Surgical Protocol
The Power surgical protocol was developed to provide
surgeons with indications on how to choose the most suitable
instruments for implant site preparation, depending on the
type of bone.
However, it is the duty of the surgeon to apply the most
appropriate surgical protocol on the basis of his/her experience
and following a thorough assessment of the clinical situation
of the individual patient.
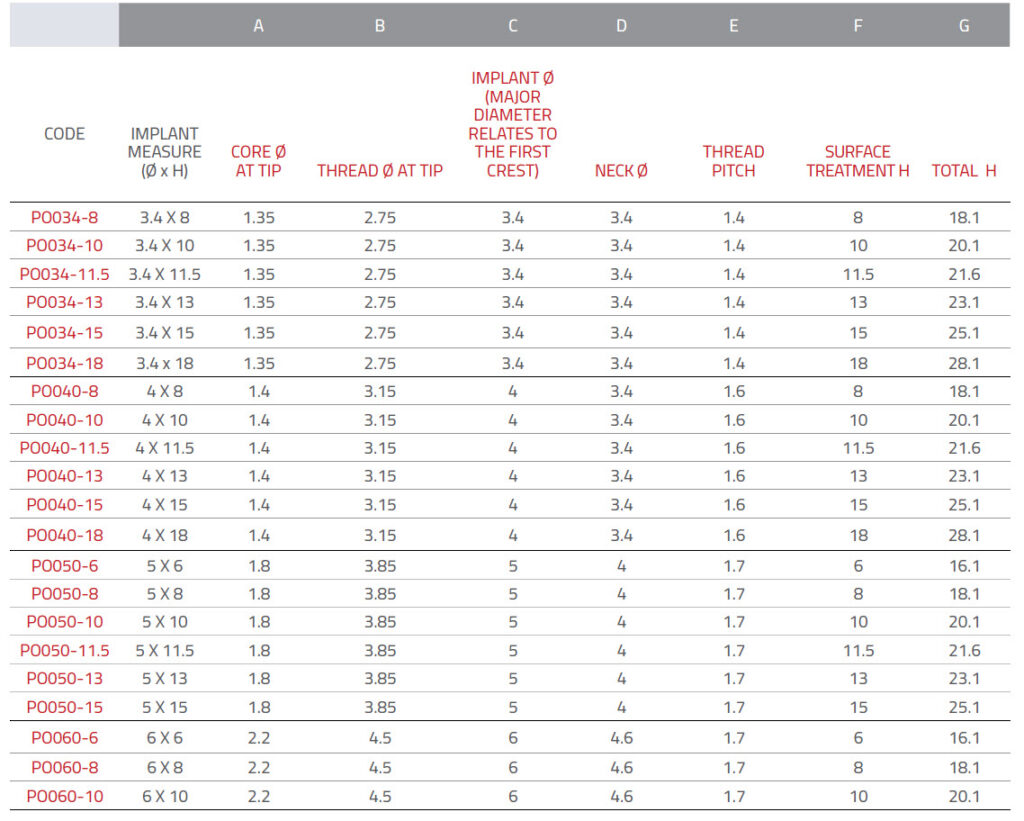
For the preparation of the implant site, IML has developed
cylindrical drills with a tapered tip and depth marks in
accordance with the length of the implant; they can be used
with drill stops.
RECOMMENDED IMPLANT INSERTION
TORQUE: 45 Ncm
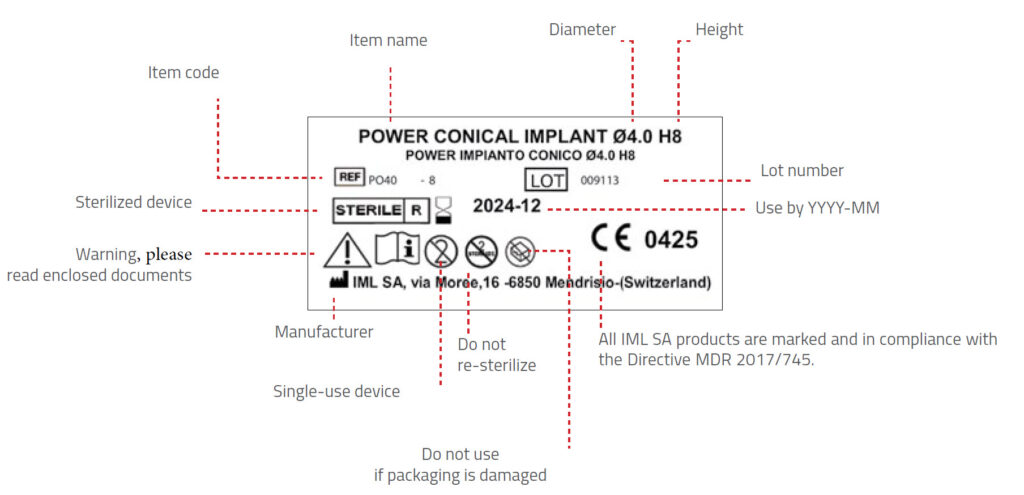
Packaging
IML packaging process is performed in compliance with the standards set by the MDR 2017/745 Directive, which
guarantee the sterilisation shelf-life.
All the IML implants are sterilised by beta rays.
Implants are packaged in a vial that, in turn, is placed inside a plastic container closed by a cap with safety seal and
bearing a label with the identification data of the implant. Then the plastic container is placed inside a cardboard
box bearing the same label. Further two copies of the label are into the cardboard box, to be placed on the implant
passport and on the patient’s medical record sheet